One group, two locations - bridging the gap between academia and industry
About Katja
Katja is the Professor of Medical Technologies and Regenerative Medicine and Director of the Institute of Biomedical Engineering (IBE) at the Faculty of Medicine of the University Hospital Tübingen, as well as the Study Dean of the Medical Technology program at the University of Tübingen.
IBE
Katja is the Director of The Natural and Medical Sciences Institute (NMI) at the University of Tübingen in Reutlingen
NMI Reutlingen
Our artwork done in collaboration with Wiley was selected for the issue cover ...
Nidogen-1 mitigates ischemia and promotes tissue survival and regeneration ...
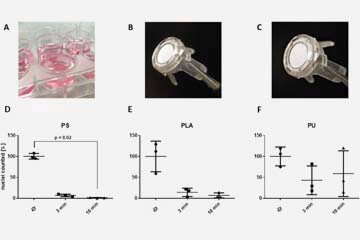
Macrophage retrival from 3D biomaterials: A detailed comparison of common dissociation methods ...