Semilunar valve leaflets have a well-described trilaminar histoarchitecture, with a sophisticated elastic fiber network. It was previously proposed that elastin-containing fibers play a subordinate role in early human cardiac valve development; however, this assumption was based on data obtained from mouse models and human second and third trimester tissues. Here, we systematically analyzed tissues from human fetal first (4-12 weeks) and second (13-18 weeks) trimester, adolescent (14-19 years) and adult (50-55 years) hearts to monitor the temporal and spatial distribution of elastic fibers, focusing on semilunar valves. Global expression analyses revealed that the transcription of genes essential for elastic fiber formation starts early within the first trimester. These data were confirmed by quantitative PCR and immunohistochemistry employing antibodies that recognize fibronectin, fibrillin 1, 2 and 3, EMILIN1 and fibulin 4 and 5, which were all expressed at the onset of cardiac cushion formation (~week 4 of development). Tropoelastin/elastin protein expression was first detectable in leaflets of 7-week hearts. We revealed that immature elastic fibers are organized in early human cardiovascular development and that mature elastin-containing fibers first evolve in semilunar valves when blood pressure and heartbeat accelerate. Our findings provide a conceptual framework with the potential to offer novel insights into human cardiac valve development and disease.
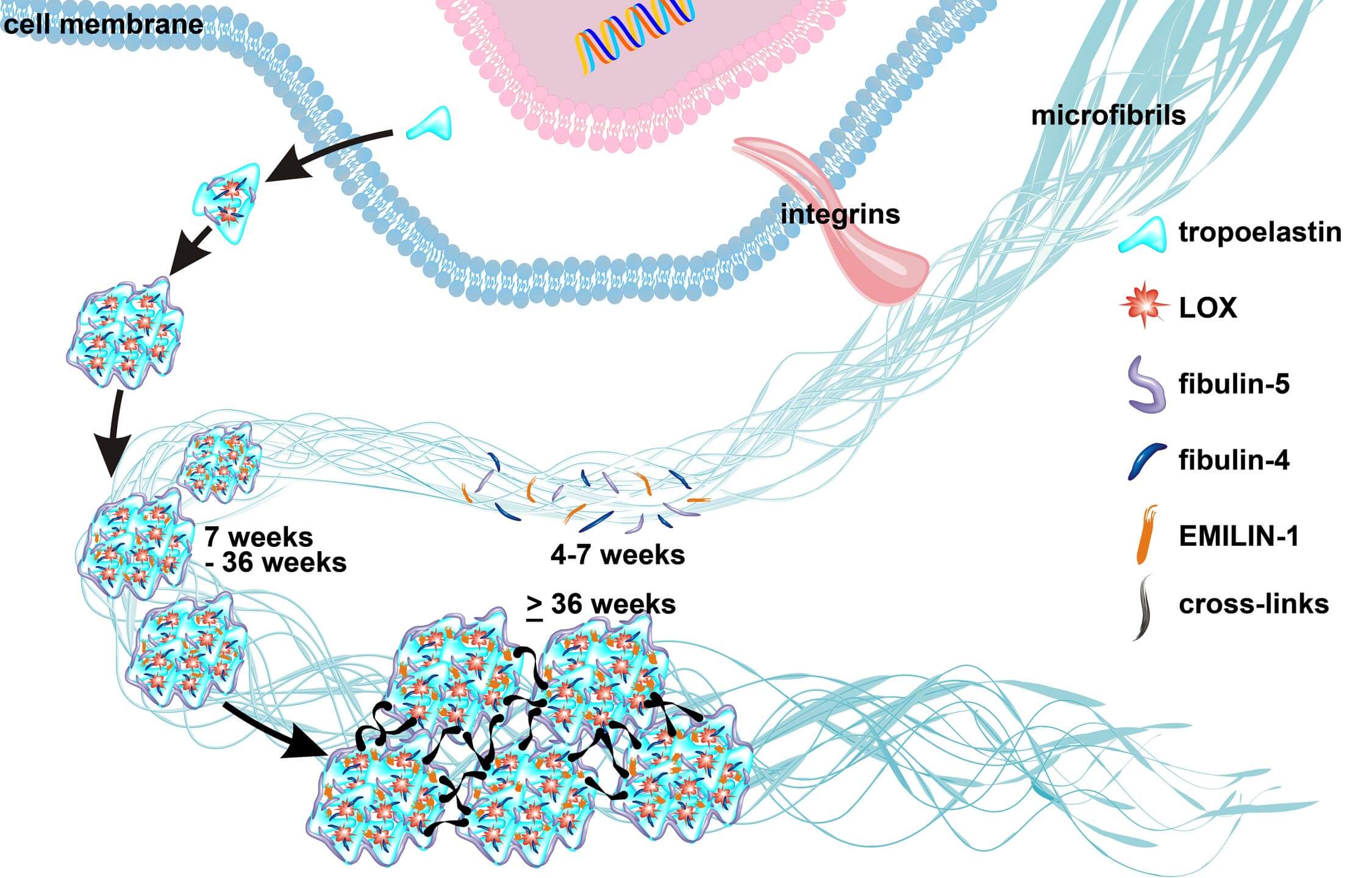
Elastogenesis
Islet-1 Niches
During development, extrinsic signals from the environment are transduced and converted to intrinsic changes in gene expression that specify the identity and function of cells. Gene regulation gives the cell control over structure and function, and is the basis for cellular differentiation and morphogenesis. These critical developmental processes take advantage of cooperation of transcription factors and epigenetic modifiers, yet very little is known regarding how these events are initiated and regulated. Various niche factors including ECM proteins, growth factors and cytokines, as well as the physiochemical and biomechanical environment, act on progenitor cells to alter gene expression, induce cellular proliferation and differentiation, and define how cell fate is stabilized and segregated from alternative fates. When misregulated, these environmental cues may lead to pathologies by inducing aberrant function in stem cells or other targets. If we are able to identify these critical developmental processes, we can then use it to stimulate regenerative strategies or to provide insight into disease pathologies.